Last time, I explained why I think the “sex spectrum” is a barren idea. However, the proof of the pudding is in the eating, so let’s look at what its advocates say. In the recent paper “Multivariate Models of Animal Sex”, McLaughlin et al (hereafter dubbed “McLaughlin” with plural pronouns to reflect the paper’s multiple authorship) assert that sexual identity is too multidimensional to measure on a single scale. They got it published in Integrative and Comparative Biology, a respectable scientific journal.
The authors argue that since sexual identity results from a mixture of genes, hormones, anatomy, and behavior, it’s obsolete to reduce sex to egg or sperm production; instead, sex should be considered a multifaceted construct. Their paper summarizes articles that question the binary sperm/egg definition before wheeling in the special cases that purportedly break it.
First, it’s worth noting that “Multivariate Models of Animal Sex” is a theoretical article, which means that it summarizes the conventional wisdom before offering a fresh perspective. In contrast, research articles, the gold standard of science, provide new evidence either for or against a novel hypothesis. Since McLaughlin don’t have any evidence at hand, they must demonstrate how their model solves puzzles better than its competitors, which means that their paper hinges on how well it describes the current paradigm. As we will see, their paper achieves neither goal.
There’s another problem with theoretical summaries: How can the reader be certain that the authors aren’t cherry picking the articles they’re surveying? Scientists address this problem through systematic reviews and meta-analyses to ensure that they’re including and correctly interpreting all relevant papers on the topic1. McLaughlin have instead chosen a narrative-style review that lacks an objective way to corral and evaluate prior studies. Although McLaughlin includes a meta-analysis towards the end of the paper, it only concerns how scientists’ views on gender shape biological terminology. The authors’ main claim rests on anecdotes.
Now, I don’t blame the authors for using a narrative review since both systematic reviews and meta-analyses are better suited for addressing quantifiable questions such as, “Do gun-control policies reduce violent crime?” Nevertheless, researcher bias doesn’t evaporate simply when researchers tackle more conceptual ideas. I suspect, in fact, that the bias is magnified — especially for politically charged topics such as sexual identity.
This paper confirms my fears. Let’s see why.
The Biological Players
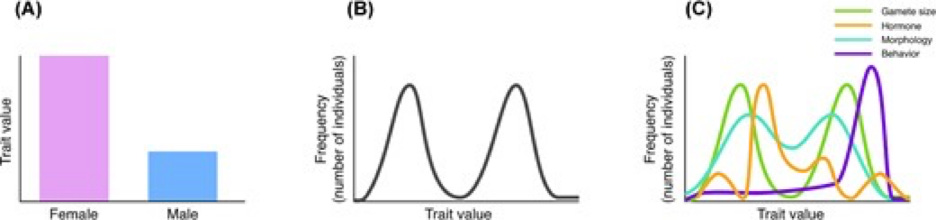
McLaughlin contrast three models of sex: the strict binary, the bimodal model, and the multivariate model. The authors illustrate each model followed with a caption:
Three models of sexual phenotypes, demonstrated by their distribution across a hypothetical population: (A) A strict binary, for which all individuals are unambiguously grouped into one of two categories. Whereas some traits, such as gamete size, operate this way, a binary does not accurately model the distribution of most phenotypic traits. (B) A bimodal model, with most individuals falling around two peaks, or means, of a single sexual trait with continuous variation. (C) A multivariate model of sex as a collection of traits, represented as individual lines, that contribute to the overall sex phenotype. Each trait has its own distribution, which may or may not be bimodal, or coincide with other traits.
The Set-Up
As the reader can see, each model portrays sexual identity differently. However, while the binary and bimodal models represent “male” and “female” as meaningful categories, the multivariate model depicts male and femaleness as hazy entities whose shape depends on the tool used to measure them. Sort of like quantum physics without the numbers or experiments.
The authors emphasize this fuzziness:
Individuals may possess different combinations of chromosome type, gamete size, hormone level, morphology, and behavioral roles, which do not always align or persist across an organism’s lifespan (Karkazis 2019; Griffiths 2021). Reliance on strict binary categories of sex fails to accurately capture the diverse and nuanced nature of sex.
According to the authors, if sex is a discrete concept, then all ways of analyzing it should yield the same result. Yet different measurements produce conflicting signals, implying that sexual identity results from a heady cocktail of environmental and genetic factors. Thus, a multimodal approach is needed to define sex properly.
Now, it’s certainly true that an individual’s chromosomes don’t always predict his or her anatomy; for example, women with Swyer syndrome often have a functioning uterus and Fallopian tubes despite possessing the male-like XY chromosome pair. Unfortunately, since these subjects have undeveloped “streak” gonads that produce neither eggs nor estrogen, they often need hormone therapy to reach puberty. Likewise, XX males have their male-determining sry gene translocated from a Y to an X chromosome during the first phase of meiosis. This translocation gives this X chromosome the ability to make a penis and testicles, usually the y-chromosome’s job (although some males possess ambiguous genitalia).2 Chromosomes don’t always tell.
McLaughlin remind us that chromosomes can be even more baffling; for example, some African Cichlid fish determine sex through two pairs of sex chromosomes that create three types of females and one type of male. Some species even establish sex through complex interactions among multiple loci.
Surely the sexual identity that results from these combinatorial networks must be equally complex?
This assumption may be reasonable, but the evidence says otherwise. With the exception of some plant species, the organisms emerging from these byzantine systems produce either eggs or sperm, full stop. It turns out that complex processes can generate simple outputs. As Moore and Roberts explain in an article about polygenic sex determination:
The traditional view of primary, gonadal sex is of a binary trait — each individual is either male or female. With multiple interacting loci determining primary sex, there are as many genetic sexes for a given group as there are possible combinations of sex determination loci. In known cases of PSD [Polygenic Sex Determination] in animals, primary sex remains binary, but evidence suggests that genotypically different individuals of the same primary sex can have differential reproductive success, as mentioned above. Thus, even though primary sex may be binary in these cases, PSD may produce different classes within a single sex, or individuals of the same primary sex with strikingly different secondary sexual characteristics. In some plants, PSD systems may produce what could be considered more than two sexes.
[all edits and bolding are mine unless stated otherwise]
In other words, although sexual determination takes multiple paths, all roads lead to the binary, at least for animals.3 A subtype of male or female does not constitute a new sex. If it did, researchers wouldn’t be able to note when an individual’s sexual behavior clashes with his identity. Take orangutans. Some orangutan males fail to develop the cheek pads and throat sacks that would make them irresistible to females. A few gender activists claim that these eternal juveniles represent a new sex. Yet the underdeveloped orangutans can and do produce offspring with female orangutans, making them unequivocal males. Treating underdeveloped males as a separate sex that just happens to produce gametes indistinguishable from “true” males makes things more complicated without providing additional benefit, tossing Occam’s razor into the compost heap.
McLaughlin’s Flawed Tale
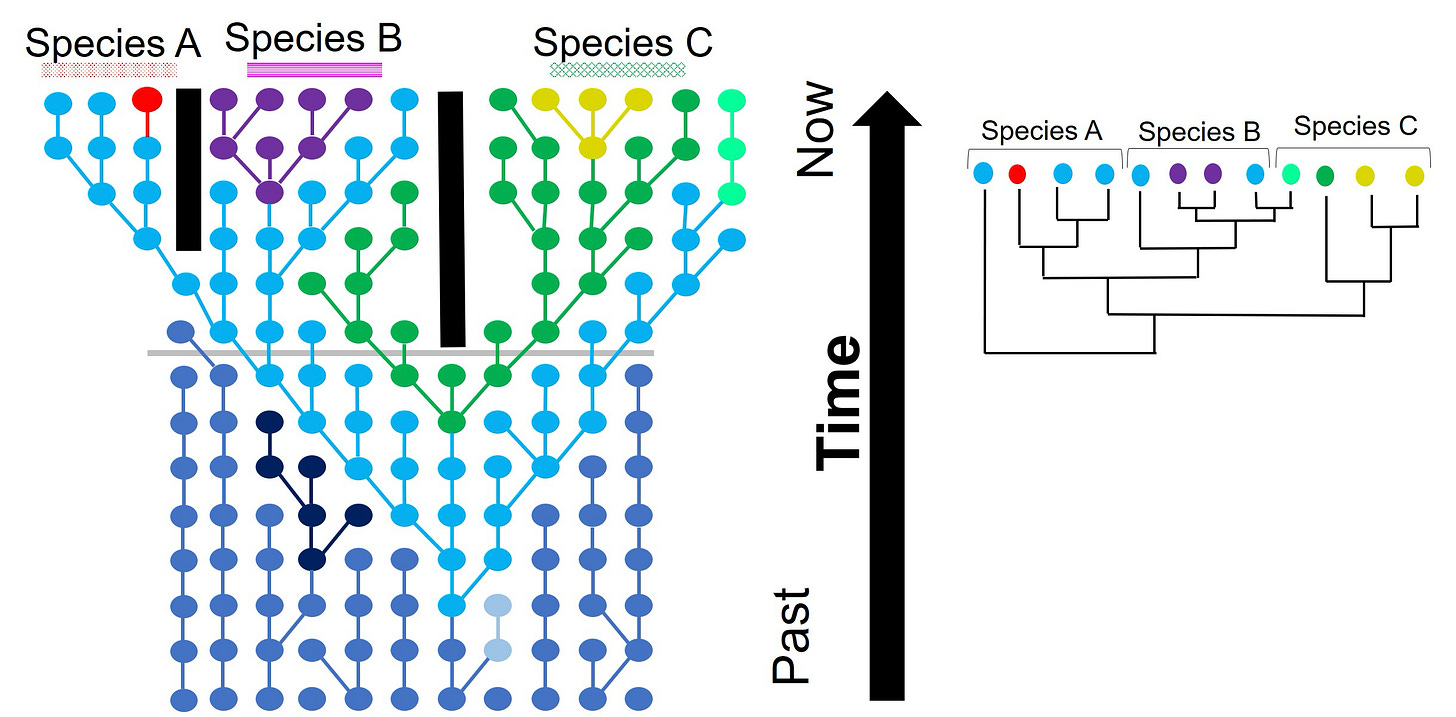
McLaughlin’s definition faces an even bigger obstacle: demanding that males and females be distinct at all levels of biological measurement before permitting them to be grouped as separate sexes is, quite frankly, starkers. This rule would make most biological classification impossible. To see why, let’s turn to evolutionary phylogeny, the science of building species trees.
You’d think that building evolutionary trees would be a simple task: just sort the critters by the similarity of their DNA sequences.This works for the most part. It turns out, though, that rapidly budding species often experience “incomplete lineage assortment”, which means that certain gene variants, or “alleles”, appear in a checkerboard pattern across unrelated species. This can occur if the ancestral population has multiple alleles floating around or if descendants from different branches mate with each other after they separate from their common ancestor.
Illustration from Sean Buckley, Dr Yuma (Jonathon) Sandoval-Castillo and Dr Catherine Attard
Among primates, Rivas-González et al have discovered that
the level of incomplete lineage sorting in the human-chimp-gorilla branch adds up to ~30%, which means that, even though our closest primate relatives are chimps, 15% of our genome resembles more the gorilla than the chimp genome, and another 15% groups the chimp with the gorilla first.
It turns out that one can’t invariably derive a consistent species tree from independent genetic sequences. In fact, biologists have long known that gene trees don’t always match trees built from anatomy, let alone the real species tree. Does that mean that there are no “true” ancestor-descendant relationships between chimps, gorillas, and humans, that it’s all down to which genes the researcher selects? A nuanced view, perhaps, but one that’s far from reality.
Ok, ok, so tracing the genealogy of closely related species can be tricky. But surely everyone would still agree that humans, chimps, and gorillas are more closely related to each other than, say, humans are to birds?
According to McLaughlin’s guidelines, however, we’ve only just begun. We must now group organisms on the hormonal, morphological, and behavioral levels. Let’s examine animal behavior. The ability to use tools in novel ways is certainly a valid way to sort animals by how clever they are. And, as expected, humans and chimps share this ability. It turns out, however, that cockatoos also possess this trait, throwing a spanner in the works:
Cockatoos can combine multiple tools to complete a complex task, a feat scientists previously thought only chimpanzees and humans could do, a new study reveals.
Tool use has been observed in a handful of animals including chimps, gorillas, orangutans, sea otters, dolphins, octopuses and crows, as well as some cockatoos. But in most cases, these animals can only use a single tool to complete simple tasks.
Scientists first discovered that Goffin's cockatoos (Tanimbar corella) could use tools by accident, when captive cockatoos used sticks to reach nuts trapped behind fencing in a laboratory setting. Since then, the brainy birds have been taught to play a rudimentary game of golf, where they use a stick to sweep a ball into a hole.
So, let’s assume that the researchers are correct and that cockatoos, chimps, and humans are the only species that can make advanced tools. Would this not imply that, with respect to creating complex tools, humans and chimps are more closely related to cockatoos than either ape is to gorillas?
Even if we try to rationalize this odd arrangement by claiming that primatologists have shortchanged the tool-making ability of other apes, then we’d have to consider the possibility that distantly related species such as octopuses (octopodes?) might also possess this skill, further muddying the ancestral waters. And that’s ignoring the fact that cockatoos can mimic a few words — and potentially understand them in their own birdy way. The main point is that a genealogical tree based solely on animal behavior is a mess that can’t be fixed by tossing extra behaviors into the hopper. A behavior tree certainly wouldn’t reflect a species’s true heritage.
Fortunately, no evolutionary phylogenist would use a multimodal approach to establish ancestry. They wouldn’t argue that since trees built independently from hormonal, genetic, anatomical, or behavioral characteristics don’t match each other, then ancestry is an artifact of the tools used to measure it. They know, for example, that similar environments can build similar-looking creatures that are, in reality, distantly related. What a phylogenist would do is select genetic and anatomical characters that diagnose ancestry and build trees based on those characters4. For example:
Notice that whales are grouped with hippos inside the artiodactyl clade, which includes deer, pigs, and camels. Biologists are well aware that whales and dolphins don’t look like deers and pigs; however, multiple genetic analyses finger these species as close relatives. Genes are usually more reliable than anatomical traits at tracing ancestry, partly because gene trees are more quantifiable and partly because whales inhabit environments that have selected for different body shapes. It turns out that Bambi and Flipper have more in common than you’d think. Furthermore, this seemingly odd grouping makes a prediction borne out by the fossil record:
This hypothesized [whale-artiodactyl] phylogeny leads us to predict that ancient whales should share some characters with their close relatives. The close relatives of whales have a type of ankle called a double pulley ankle, so we would expect that ancestral whales would also have a double pulley ankle.
And in fact, recent fossil discoveries have borne out that prediction. Scientists found ancient whales with hind legs and pelvises: these whales had the same kind of double pulley ankle bone that modern pronghorns, camels, cows and hippos have.
It’s almost as if “ancestry” is a biological reality instead of definitional taffy that can be stretched or squashed at the whim of political activists. Phylogenies reflect ancestry, not wordplay. And since most animals result from the fusion of distinct gametes, defining an individual’s sex by its gametes simply acknowledges the way in which ancestry unfolds over time. In order to understand evolution, one must have clear idea of how it works. This makes the traditional definition of sex a fruitful one.
The Shut-Out: Or How to Ignore the Perfect Solution
This lengthy counterexample shows how McLaughlin’s multimodal approach withers outside of its ideological hothouse. But let’s lower our expectations: Do these authors accurately portray the science of sex? After all, the chief duty of a research summary is to….summarize research. A humble standard, but an honest one.
Alas, McLaughlin’s article fails even this modest test. Take this passage from “Case Study 2: The evolutionary consequence of more than two sexes”:
How does the number of operative sexes influence the evolution of a species? White-throated sparrows (Z. albicollis), have been described as “the bird with four sexes,” with both tan and white-stripe morphs occurring in ZZ and ZW individuals. This species mates disassortatively by color morph, with tan stripe morphs predominantly mating with white-stripe morphs (Hedrick et al. 2018). The more aggressive white-stripe morph has a large inversion on chromosome 2 that functions as a “supergene,” which tan-stripe individuals lack (Tuttle et al. 2016; Falls and Kopachena 2020; Maney et al. 2020; Fig. 2G). These morphs are further associated with differences in testosterone and estrogen (Maney and Küpper 2022). Population persistence as a whole requires that all four morphs be present (Campagna 2016; Maney et al. 2020). A similar system occurs in hybrid Pogonomyrmex ants, where each colony requires three sexes to operate, and four to persist beyond a single generation (Parker 2004).
Let’s focus on the ants first.
The Hook: How Many Sexes Do Ants Need?
Pogonomyrmex ants, also known as harvester ants, live in the southwestern U.S. Two species known as Pogonomyrmex rugosus (rough harvesters) and Pogonomyrmex barbatus (red harvesters) have recently interbred to form hybrid lineages. Scientists refer to these lineages as H1, H2, J1, and J2 after the Hidalgo and Junction cities in New Mexico. If you recall your myrmecology (and who doesn’t?), ant societies are divided into several castes, or social divisions:
1. Queen: The queen is the central figure in an ant colony. Her primary role is to lay eggs and ensure the colony’s growth and survival. The queen is typically larger than other ants and has the ability to reproduce.
2. Workers: The majority of ants in a colony are workers. They are responsible for various tasks such as foraging for food, taking care of the brood, building and maintaining the nest, and defending the colony. Workers are typically smaller in size and have specialized features for specific tasks.
3. Soldiers: Some ant species have a specialized caste of soldiers. These ants have larger heads and powerful jaws, enabling them to protect the colony from threats and intruders. Soldiers may also assist in tasks such as nest defense and brood care when needed.
4. Drones (Males): Male ants, also known as drones, have the sole purpose of mating with the queen to ensure the colony’s reproduction. They have wings and are responsible for leaving the colony during the mating flight.
Each type of worker provides a vital role, but workers are the spit holding the colony together. Without someone to gather the food and care for the queen’s offspring, the entire social system collapses. Colonies need workers.
These hybrid ants have evolved a strange reproductive triangle: matings between a queen and drone of the same lineage (either H1 with H1 or J1 with J1, depending on location) will produce a new queen, while a cross-lineage mating (i.e. H1 with H2 or J1 with J2) yields worker ants. Therefore, a viable colony requires input from two male lineages. If a queen exclusively mates with a male of the opposite lineage, she will only be able to produce workers and drones (drones arise from unfertilized eggs, so they don’t need two parents). This colony, lacking a queen, will die after a single generation. Conversely, a queen who sticks to males of her own lineage will only produce a colony of drones and queens. Without workers, her colony perishes before it can get off the ground.
McLaughlin cites Parker’s 2004 paper to show that these hybrid ants require four sexes to survive beyond a single generation. Indeed, Parker argues that
The obligatory interdependence of gametic types across the two gene pools also has important ramifications for the persistence of the population as a whole. According to the stability definition above, to classify a system with more than two sexes, the gametes must be divisible into more than two independent sets, such that the loss of any one of the sets results in the extinction of the entire system. SSH populations meet this criterion. Losing any one of the four gametic types (yellow sperm or egg, blue sperm or egg in Figure I, Box 1) will result in the collapse of the entire system.
If we operatively define a sex as “a breeding group needed to keep the population going”, then sure, you could define H1 and H2 males as two “sexes” and the drone-queen complex as four sexes. For that matter, I could classify myself as a dual-threat sexual/asexual reproductive unit with the independent gametic sets being “sperm” and “none”, respectively. Here’s my reasoning:
I need to digest food properly in order to get through the day.
My gut has multiple colonies of bacterial that help me digest food. They reproduce asexually.
I define myself as a system consisting of a primate body + gut bacteria.
Without either the primate or bacteria part, the system won’t function.
Therefore, classifying me as a sexually-reproducing organism rejects my nuanced identity as a trans-reproductive strategist.
My pronouns are “convenient/definitions”. Please respect them.
More seriously, Parker is free to define the sex of harvester ants as he wishes. However, there’s a bigger issue. Unlike McLaughlin, Parker recognizes that there’s an alternative evolutionary explanation that doesn’t require rejiggering widely-accepted definitions: social hybridogenesis. What is this phenomenon of which I speak? As the evolutionary biologists Lavanchy and Schwander explain:
Hybridogenesis is an unusual form of reproduction that is found in hybrids between different species. It involves the selective transmission of one of the parental genomes, while the other one is renewed by mating with the corresponding species. It is seen as a form of sexual parasitism, in which the hybridogenetic genome gains a twofold transmission advantage and exploits the reproductive effort of another species.
To illustrate, the authors describe a Mexican freshwater fish that is a cross between the P. lucida and P. monacha species. Surprisingly, this fish can reproduce with an entirely female population! Here’s how it manages this trick.
First, a female lets a male from the closely-related P. lucida species fertilize its genetically pure monacha eggs. The eggs then block any male sex-determining genes that might be present, leading to strictly female lucida-monacha hybrids. When the resulting offspring are ready to lay their own eggs, they flush out the male chromosomes, ensuring that their eggs contain only monacha genes. These genetically pure eggs are ready for the next lucida sucker’s male’s sperm. Rinse and repeat.
Since males from the lucida species don’t pass on their genes to these ramblin’ roses, their contribution is wasted. However, this arrangement lets the parasitic monacha species avoid the awkward process of sorting incompatible chromosomes during meiosis.
With the background established, let’s turn to the main point: do Lavanchy and Schwander consider the Pogonomyrmex reproductive arrangement a form of hybridogenesis? You bet:
Finally, the third type of social hybridogenesis differs more extensively from classical hybridogenesis, and is found, for example, in the genus Pogonomyrmex. Here, [as with the little fire ant], workers are also produced through hybridization between two lineages, but new queens are produced sexually, instead of via parthenogenesis, through within-lineage matings. While there is still only one type of genome transmitted to future generations [remember, workers are sterile — Fisk], this genome is not asexually transmitted, which is expected to have a positive effect on the long-term persistence of this form of hybridogenesis.
Sara Helms Cahan, who has written extensively on these ant hybrids (she has coauthored papers with Parker, and Parker’s 2004 paper includes her in his list of acknowledgments) also interprets the Pogonomyrmex strategy as social hybridization, noting that
An extreme form of genetic caste determination is “social hybridogenesis,” in which co-occurring genetic lineages obligately interbreed to produce workers, whereas daughter queens are of pure-lineage ancestry. In this study, we tested the hypothesis that social hybridogenesis in the genus Pogonomyrmex resulted from one or more interspecific hybridization events, and if so, whether individual lineages were of hybrid ancestry.
But why does social hybridogenesis better explain this odd hybrid ant arrangement than Parker and McLaughlin’s four-sex model? For one, social hybridogenesis doesn’t produce a paradox.
If we interpret this reproductive triangle as a multi-sex system, then we’d have to explain why evolution would create two extra “sexes” whose offspring are destined to be sterile — a dead-end strategy that defeats the purpose of making extra sexes in the first place! In contrast, viewing this arrangement as reciprocal sexual parasitism makes perfect sense and fits with examples like the monacha fish. To be sure, the sperm of H1 and H2 ants will differ, as will sperm from J1 and J2 ants, but this is because the ants belong to different subspecies. Perhaps the extra hybrid vigor compensates for the lower gametic compatibility? After all, it’s not as if the colony has to concern itself with the offspring of workers.
Cahan’s quote also illustrates how viewing this reproductive strategy through the lens of social hybridogenesis generates useful follow-up questions. How exactly did these hybrid ants form? How did this cross-breeding strategy evolve when forming worker ants through more traditional methods such as maternal hormones and environmental cues seem more efficient? How many shots of Raid does it take to kill these ants if you encounter them in your kitchen?
McLaughlin don’t say how their model will answer such questions, which is OK. However, McLaughlin should at least acknowledge that other perspectives exist. Failing to do so leads to bad review articles, of which this represents a shining city on a hill.
The Shut-Out: Part Deux
Even when acknowledging other points of view, McLaughlin often omit critical details. For example, they admit that biologists traditionally classify males from different lineages as “subtypes” of males rather than different sexes, but leave out why this is done. Here’s McLaughlin’s claim:
It is typical to frame such systems as having subtypes of two sexes—in this case, three males and one female. But if we are willing to consider the white-throated sparrows and Pogonomyrmex systems as having more than two sexes, why not apply a similar framework to the ruff [a type of shorebird] and other polymorphic systems (Galeotti et al. 2003; Roulin 2004)? Our binary framing of sex in these systems may interfere with understanding their evolutionary trajectories.
However, since male subtypes can all mate with females to produce fertile, healthy offspring, it makes sense to consider them part of a single sex. As for ruffs, the fact that some alleles may give some male shorebirds a showy plumage while other gene variants produce plainer looking or even feminine males is interesting, but hardly revolutionary. Evolutionists even have a term for this alternative reproductive strategy: the “sneaky f*cker” gambit:
Leonardo Campagna elaborates:
Male Ruffs come in three types: most (about 80 to 95 percent) have fancy plumes and an appetite for fighting. Scientists call them “independents.” Another 5 to 20 percent of males, known as “satellites,” take a subsidiary role. They’re not as colorful but display their feathers alongside the independents and help attract females to the lek. In return the independents tolerate them—and occasionally permit satellites the chance to mate with a female.
Then there’s an entirely different approach, used by a very rare third kind of male (less than 1 percent) known as a “faeder” (after the Old English word for “father”). Faeders don’t fight, and they don’t sport any colorful feathers at all. In fact, they look just like a female Ruff, which allows faeders to sneak onto the lek without attracting unwanted attention from the fight-loving independents.
Even though faeders look female, their testes are much larger than an independent’s. The faeder’s strategy involves waiting until a female signals to a displaying independent that she’s ready to mate. At this critical time, the faeder quickly ducks in and mates before the independent has a chance—deceiving both the independent male and the female. If this sounds sneaky, it is. Behavioral ecologists actually refer to it as the “sneaker male” approach. It’s widespread across the animal kingdom, especially in fishes.
This evolutionary strategy explains how polymorphic traits can evolve among males. You’d think a review article might explain that there’s an easily accessible and testable hypothesis that solves the riddle posed by the article. And you’d be very, very wrong.
The Sting: Building the Perfect Straw Man
Restoring the missing context reveals the biggest problem of all, which is that the authors seemingly believe that disproving a hypothesis arising from the binary definition of sex is equivalent to refuting the definition itself. This hypothesis, known as the Darwin-Bateman paradigm5, claims that differently-sized gametes lead to the evolution of sex-stereotypic behaviors. McLaughlin explain that
Scientific notions of how animals “should” adaptively behave originate with Darwin in the Victorian era (Darwin 1871). The Darwin–Bateman paradigm posits that “traditional” sex roles arise from anisogamy, such that mate competition is stronger in the sex with the smaller gametes (Bateman 1948; Dewsbury 2005). Trivers (1972) extended this paradigm with the hypothesis that gametic investment drives postzygotic parental investment, which should be higher in females because of larger gamete size. Theories on the evolution of sex roles have dominated sexual selection research, but not without criticism (Gowaty et al. 2012; Ah-King 2013; Tang-Martínez 2016), as others have argued for the importance of ecology, sex ratio, and life history traits on the evolution of sex-specific behaviors (Kokko and Jennions 2008; Mokos et al. 2021; Kappeler et al. 2022). Indeed, there are many examples of competitive females and choosy males across animal groups (reviewed in Edward and Chapman 2011; Hare and Simmons 2019). Outside of mammals, many examples in fish, frogs, and birds contradict the notion that the sex with the larger gamete suffers higher mating costs and invests more resources into the next generation. For instance, male parental care has evolved repeatedly in frogs (Townsend et al. 1984; Gross 2005; Furness and Capellini 2019).
McLaughlin mistakenly conclude that the ability to sort sexes into male and female hinges on the truth of Darwin-Bateman hypothesis:
Some readers may consider our examples of sexual diversity as rare anomalies—exceptions to the evolutionary rule, which do not require a novel framework to explain. We acknowledge some variations in sexual phenotypes may be relatively less common in animals, but that does not minimize their importance in our understanding of evolution. When we regard diversity as a deviation from the norm, we ignore its successful role in generating novel solutions to evolutionary challenges. Our frameworks of sex need to accommodate and embrace this diversity, lest we fall prey to a deterministic model of evolution, where the ideal endpoint is always binary.
They repeat this claim here:
Binary expectations of sexual phenotypes are often met with exceptions, and one classic example are birds with polyandrous mating systems, including black coucals (Centropus grillii), spotted sandpipers (Actitis macularius), buttonquails (Turnicidae), and northern jacanas (Jacana spinosa). In these systems, individuals with larger gametes typically face stronger sexual selection for mating opportunities (Fritzsche et al. 2021), decoupling sexual phenotypes from anisogamous expectations. These systems are often called “sex-role reversed” because they defy “traditional” expectations of sex roles historically found in mammals—male competition and female parental care. However, sex role terminology imposes binary categorization, limiting our understanding of continuous variation in phenotypes (Ah-King and Ahnesjö 2013). As we describe below, polyandrous mating systems are not simply the “reverse” of polygynous mating systems, but rather, they are novel evolutionary outcomes to be studied in their own right, not solely in relation to a polygynous foil. We propose that studying so-called “exceptional” systems can help reveal unexamined routes of phenotypic evolution, expanding the conceptual framework for how we study sexual diversity.
and here:
These case studies demonstrate that breaking the sex binary—and expanding to a multivariate model of sex—is a useful framework for understanding animal ecology and evolution. Each of these animal systems possesses a different set of genetic, endocrine, morphological, and behavioral sexual traits, which do not fit neatly into binary distributions. Once we detach our assumptions of binary sex classifications, we can ask more expansive questions such as “how does variation within and among these sexual traits shape ecological and evolutionary processes?”
If McLaughlin just wanted to bash the Darwin-Bateman hypothesis, they’re late to the party. But their complaints are to little avail. As their own summary of the Darwin-Bateman hypothesis reveals, the existence of two gametes does not logically entail that all sexual traits are binary. If the binary depended on the Darwin-Bateman model, then researchers who dispute this model would have to reject the binary classification. Yet even critics of the D-B hypothesis have no problem agreeing on what constitutes a male ant, hyena, anglerfish, or shorebird.
Why? Perhaps it’s because most biologists recognize that selection doesn’t change each biological level in lockstep. Perhaps they recognize that although the Darwin-Bateman paradigm depends on the binary definition of sex, the converse isn’t true. Therefore, observations that trouble the D-B model don’t necessarily refute binary classification. In order to overturn the binary approach, McLaughlin must show why redefining “sex” is necessary to solve puzzles arising from exceptions to the Darwin-Bateman model. Pointing out that the “binary” (actually, the D-B model) fails for this or that species is not telling scientists anything useful and consequently provides no reason to abandon a useful way to categorize sexes. Even worse, their multimodal approach clashes with hypotheses like as the sneaky f*cker gambit that do explain exceptions to the Darwin-Bateman paradigm, or, as McLaughlin dub them, “variation within and among these sexual traits”. Alternative explanations that McLaughlin’s paper then proceeds to ignore.
So, let’s revisit the white-throated sparrows to check how the multimodal approach handles the exceptions it brandishes. Remember McLaughlin’s earlier claim?
How does the number of operative sexes influence the evolution of a species? White-throated sparrows (Z. albicollis), have been described as “the bird with four sexes,” with both tan and white-stripe morphs occurring in ZZ and ZW individuals. This species mates disassortatively by color morph, with tan stripe morphs predominantly mating with white-stripe morphs (Hedrick et al. 2018). The more aggressive white-stripe morph has a large inversion on chromosome 2 that functions as a “supergene,” which tan-stripe individuals lack (Tuttle et al. 2016; Falls and Kopachena 2020; Maney et al. 2020; Fig. 2G). These morphs are further associated with differences in testosterone and estrogen (Maney and Küpper 2022). Population persistence as a whole requires that all four morphs be present (Campagna 2016; Maney et al. 2020).
McLaughlin tout the sparrow case study because they believe that interpreting different-colored morphs as different sexes sheds light on overlooked evolutionary processes. They argue that6
As an ecological consequence, the overall greater niche breadth of polymorphic populations is hypothesized to confer greater capacity for range expansion, less susceptibility to environmental change, and a larger buffer to extinction risk (Forsman et al. 2008). Evolutionary consequences of multimodal morphs include punctuated patterns of trait evolution, greater mobility on the adaptive landscape, and increased diversification rates (West-Eberhard 1986; Corl et al. 2010; Hugall and Stuart-Fox 2012; Brock et al. 2022a). New species could form from different morphs if they become reproductively isolated or fixed within a population (West-Eberhard 1986; Gray and McKinnon 2007; Seehausen et al. 2008; Corl et al. 2010; McLean and Stuart-Fox 2014; Brock et al. 2022c). In addition to facilitating speciation, character release associated with morph fixation could produce punctuated accelerations of morphological change (Eldredge 1976; West-Eberhard 1986; Corl et al. 2010). Thus, the ecological differences that emerge from intrasexual polymorphism are a crucial component of biodiversity within a species, which has far-reaching consequences for population persistence in a rapidly changing world. Collapsing intrasexual polymorphisms into a female–male binary erases extensive multivariate phenotypic variation (Fig. 4), including its ecological and evolutionary consequences.
Certainly, biologists should understand how different morphs evolve and what adaptive advantages these arrangements bring. However, McLaughlin don’t explain how dividing morphs into two sexes “erases” phenotypic variation unless one confuses the binary definition of sex with the Darwin-Bateman hypothesis about the distinct roles each sex should play. Additionally, discussing rare reproductive strategies probably isn’t the best way to understand how most reproductive strategies evolve:
A comprehensive review of 1116 assortative mating examples in animals (Jiang et al. 2013) documented many examples of positive-assortative mating but very few examples of negative-assortative mating, particularly in populations of vertebrates.
However, there’s another angle to examine. Researchers like Tuttle et al (2016) find the mating strategies of white-throated sparrows fascinating because the inverted chromosome that causes the birds to mate with different morphs is apparently behaving like a sex chromosome. Aha, here’s our connection between sparrow stripes and sexual identity:
Chromosomes 2 and 2m in white-throated sparrows are independent of sex chromosomes but show striking parallels with patterns of sex chromosome divergence and degradation [31, 32]. Indeed, because of disassortative mating based on both chromosomes 2 and 2m and the W and Z sex chromosomes, the species operates as though there are four sexes. Sexual systems with more than two sexes are exceedingly rare among animals, and theory predicts that they are unlikely to persist for long periods of time [33]. In part, this instability arises because four- sex systems have a 2-fold increase in some aspects of reproductive effort, such as finding a mate. If such four-sex systems are truly unstable, the persistence of the inversion-based plumage morph polymorphism found in the white-throated sparrow may be transient, despite the observed fitness benefits of disassortative mating.
Are these inverted chromosomes precursors to sex chromosomes? Tuttle marshals some evidence for her hypothesis , but researchers like Campagna are more cautious:
The signature of different types of mutations can also provide information on the forces shaping molecular evolution. Tuttle et al. [4] found the supergene on 2m carries many mutations that change protein sequence, which are presumed to be deleterious and may indicate the 2m supergene is degrading. The molecular signatures on 2, however, suggest it is under purifying selection, which eliminates deleterious mutations.
This type of selection on 2 is expected to be particularly strong if 2m is degrading, and because recombination rates on 2 are low [14] (it only recombines in tan individuals). Ultimately, to better understand the genetic mechanisms that underlie the white and tan morphs, direct evidence of 2m degradation is needed. Accumulation of transposable elements and mutations that render proteins non-functional will answer the question of whether or not 2m is indeed degrading like a Y or W sex chromosome.
Unfortunately, subsequent research has shown that the inverted chromosome isn’t accumulating the random transposon hitchhikers that suggest that it’s degrading, weakening Tuttle’s analogy. As Maney et al admit:
The key to the evolution of ZAL2m is its near-constant state of heterozygosity. Inversions suppress recombination most effectively in heterozygotes; the inverted segment is free to recombine normally in individuals with two copies, but in heterozygotes, successful recombination requires a much rarer event, such as a double crossover or gene conversion (see Hoffmann & Rieseberg, 2008; Kirkpatrick & Barton, 2006; Wellenreuther & Bernatchez, 2018). The scarcity of ZAL2m/ZAL2m homozygotes means that the ZAL2m chromosome is largely devoid of recombination, and effectively undergoes asexual reproduction. As a consequence, de novo mutations can accumulate on ZAL2m independently of ZAL2. The ZAL2m sequence has, in fact, diverged (Fig. 3B) such that the two haplotypes are now 1–2% different from each other (Huynh et al. 2011; Sun et al., 2018). ZAL2m does not, however, show strong signatures of genetic degeneration. Recent analyses have revealed only a slight increase in nonsynonymous polymorphisms (Tuttle et al., 2016), and low incidence of pseudogenization and repetitive sequences, the classic markers of degeneration (Davis et al., 2011; Sun et al., 2018). Although it is not degenerating, ZAL2m is clearly differentiating in ways that can, and do, affect behavior.
A beautiful hypothesis compromised by ugly facts.
Turning to the idea of functional sex, is it true that four “sexes” are needed to keep the population intact? Maney et al suggests so:
Working with hundreds of specimens, Lowther (1961) discovered that Audubon and the field guides alike had been incorrect. Both male and female white-throated sparrows occur in two color morphs (Fig. 1A), now known as white-striped (WS) and tan-striped (TS), that are fixed throughout an individual’s lifetime. It is easy to understand why Audubon associated the coloration with male and female; almost every breeding pair consists of a WS bird and a TS bird (Lowther, 1961; Tuttle et al., 2016). WS-WS pairs and TS-TS pairs each constitute less than 1% of the breeding pairs in a population (Tuttle et al., 2016). This disassortative mating system, so far unique among birds, means that each bird can mate with only 25% of the population. Thus, the species has earned the nickname ‘the bird with four sexes’ (Campagna, 2016).
However, field observation and genetic analysis reveal that the sparrows’ finicky mating is a choice rather than a biological imperative. The low frequency of within-morph matings is partly due to the fact that the more aggressive white-striped female sparrows outcompete their darker rivals for tan males, forcing them to settle for the ne’er-do-well white-striped males. Let’s let Hedrick, Tuttle, and Gonser summarize this process:
What factors actually determine this high level of negative-assortative mating? First, the 2 phenotypic morphs differ in social behavior, including the level of parental care, aggression, and courtship (Knapton and Falls 1983; Tuttle 2003; Formica and Tuttle 2009). More specifically, W males are promiscuous at the expense of paternal care and T males contribute more to parental care (Tuttle 2003). Similarly, W females are also more aggressive and invest less in parental care than do T females (Tuttle 2003). Tuttle (1993) found that males of both morphs preferred W females and both Tuttle (1993) and Houtman and Falls (1994) found that both female morphs preferred T males. Because W females are more dominant, they outcompete T females for the preferred T males, resulting in heterotypic pairings. The remaining T females then pair with the less preferred W males.
Sexual tastes aside, these birds seem to be acting like two-sex birds. A male from either morph can fertilize a female from either morph although the male offspring are admittedly smaller and such breedings admittedly occur when the preferred morphs are unavailable. Scientists can clearly distinguish between males and females within each morph.
Still, since the inverted chromosome causes hormonal and behavioral differences, and since some biologists compare the inverted chromosome to sex chromosomes7, I suppose you could count this as partial support for the “morphs as functional sex” argument. However, it’s worth stressing that these cross-breedings are not necessary for the species’s survival.
That’s a mountain of digging for a nugget of insight.
Weird Science
McLaughlin even come up empty when they attempt to show how their framework enriches population genetics:
There are significant evolutionary consequences to having more than two operative sexes. A key parameter in population genetics is effective population size (Ne; Charlesworth 2009), which is fundamentally impacted by the number of sexes in a given population (Caballero 1994). Assuming an equal sex ratio, the absolute maximum of Ne will be equal to N/So, where N is the census population size and So is the operative number of sexes.
I couldn’t find the bolded formula in either Caballero (1994) or in a paper Caballero coauthored with Wang and Santiago in 2016. These papers did include Wright’s effective population size formula for a population that contains an unequal number of males (Nm) and females (Nf). Here it is:
As Caballero (1994) deduces (and as the reader can check for herself) the effective population size peaks at the census size “N” when the sex ratio is assumed equal (Nm=Nf). This is twice the value predicted by McLaughlin’s formula if one plugs in two operative sexes. In other words, the McLaughlin formula doesn’t even reduce to classic population genetics equations under equivalent assumptions. This assumes, of course, that we can even define an operative sex under the multimodal model. And if sex is a continuum with all or its members contributing equally to the system’s functionality8, shouldn’t we be dividing N by infinity? If so, then McLaughlin’s formula predicts that humans are already extinct. Now that’s science!
A Modest Proposal
So, what would a useful counterexample to the binary look like? Showing that animals that violate traditional sex roles have evolved similar-sized or novel gametes would be a start. I’ll even give a hand by citing a contemporaneous paper on the northern jacana, one of McLaughlin’s case studies. In this paper, Lipshutz et al argue that the that the northern jacana’s polyandric system may have altered the shape the males’ sperm:
We found that the species with greater polyandry, northern jacana, has sperm with longer midpieces and tails as well as marginally lower intraejaculate variation in tail length. Intraejaculate variation was also significantly lower in copulating males than in incubating males, suggesting flexibility in sperm production as males cycle between breeding stages. Our results indicate that stronger female- female competition for mating opportunities may also shape more intense male-male competition by selecting for longer and less variable sperm traits.
OK, it may be true that Darwin himself proposed that sexual selection can alter sperm shape, making this a very old idea9 , and it may also be true that the different jacana sperm do typical spermy things like fertilize female jacana eggs, but hey — baby steps, baby steps.
Woozle! Good God! What’s It Actually Good For?
(Activist propaganda)
Since McLaughlin love anecdotes, let’s conclude with a brief incident involving Winnie the Pooh:
Winnie-the-Pooh and Piglet start following tracks left in snow believing they are the tracks of an imaginary animal called a woozle. The tracks keep multiplying until Christopher Robin explains to them that they have been following their own tracks in circles around a spinney.[2]
This tale has led to the term “woozle”, an endeavor that characterizes a surprising amount of modern science.
Here’s how it works:
A study with a provocative claim that’s supported with slender evidence is published.
Other scientists dutifully cite the study without mentioning its weak effect size, poor experimental design, flawed logic, etc.
The study seeps into a public who’s eager to further exaggerate its claim. The study quickly evolves into conventional wisdom.
“Woozle” makes for a great verb, too: “I haven’t been woozled this bad since I attended that Ramtha seminar five years ago.”
Of course, woozling is more likely when the original paper flatters elite opinion or advances a social cause. Scientists withhold criticism because nobody wants to be the bearer of conventional wisdom in a field that rewards revolution.
However, the revolutionary spirit must be wedded to a search for empirical truth. Although moral advocacy has its place, it often yields lousy science because it dulls the critical faculties needed to mold a raw hypothesis into a useful theory. Moral conviction crowds out an activist’s judgment. Sarah Haider notes the difference between activism and scholarship:
The activist game, to sum in one sentence, is about results. The goal of a “good” activist is to achieve the ends as quickly as possible — as ethically as this might allow. Her morality is rooted in the goodness of the ends she works towards, indisputably noble means to attain them are not required.
The thinker game is about truth. The goal is to uncover reality as it is - to achieve a true map of the real world (and hopefully, to be the first to do it). Reflecting reality accurately requires honesty — with oneself and with others — and a strict adherence to principled conduct. Although all fields have some degree of competition, knowledge-building is inherently not a zero-sum game. Truth builds upon itself.
A paper this sloppy doesn’t come by accident, so it’s no surprise that McLaughlin’s concludes with an explicit call to social justice:
We recognize that TGNC and intersex people are valid regardless of the sexual diversity we find in nature. Human rights cannot be defined solely by biology—this is a prime example of the appeal to nature fallacy. However, opposition to the inclusion of TGNC and intersex people in society is often based in the language of biology. Especially in the United States, legislation targeting TGNC people is increasingly undergirded with the simplistic binary model of “biological” sex (such as OH HB454 §3129.02 2021, WV HB3293 §18–2-25d.b1 2021, MO SB22 §191.1720 2022, and TX HB672 88R §71.004.1A 2022). There is pressure for scientists to avoid making the politics of our work explicit, especially those of us who do not directly study social issues. However, our science is being weaponized to discriminate against marginalized groups (Ha et al. 2014). It is imperative that we biologists challenge the misuse and abuse of this language (Miyagi et al. 2021) and confront how our scientific models impact society (Bazzul and Sykes 2011). As biologists, it is our responsibility to dispel misconceptions and recognize the rich tapestry of diversity in nature.
Colin Wright, an activist himself, best sums up the scientist’s duty:
As biologists we should not be engaged in erasing, invalidating, or affirming people’s identities or experiences. Our job is simple: describe and explain the natural world as accurately as possible.
This seems like a good model to follow.
Just because the circus is in town, you don’t have to buy a ticket. Let’s hope the woozle ends here.
This is no small matter. Even an august professional societies can churn out misleading narrative reviews if the subject’s touchy enough, as shown here.
Unfortunately, the men can’t produce sperm since they lack other genes normally present on the Y chromosome, making them infertile.
And it’s no surprise that plants would be the ones to push the boundaries of the binary, what with their alteration of generations thingy going on.
You might be wondering if this reasoning is circular: “What are the ‘best’ characters to include in a phylogeny? Why, those characters that give the desired tree, of course!” While it’s true that some researchers have fallen into this trap, the discovery of molecular markers such as SINE and retroviral insertions allow biologists to cross-check trees built from other DNA sequences. In fact, computing techniques now allow scientists to build trees from entire genomes. SINE and retroviral phylogenies aren’t perfect, but they are hard to game.
The Darwin-Bateman paradigm argues that since females have to invest more time in producing their gametes than males do, nature has selected for choosy females and indiscriminately-breeding males. Other biologists believe the paradigm explains why females spend more time caring for their offspring.
This passage appears in the lizard case study, but the point remains the same.
Even if the inverted gene ultimately evolves into a new sex chromosome, it’s likely that it will still produce binary sexes.
To say otherwise would be bigoted. You wouldn’t want to be considered a bigot, would you? Well, would you?
Lipshutz et al mention this fact in their article.